[ad_1]
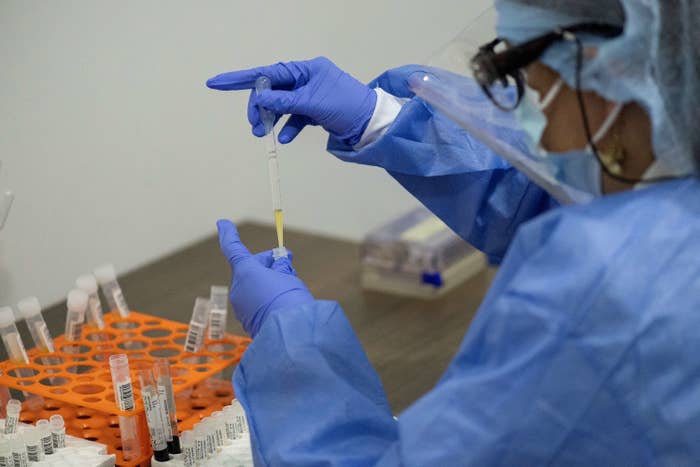
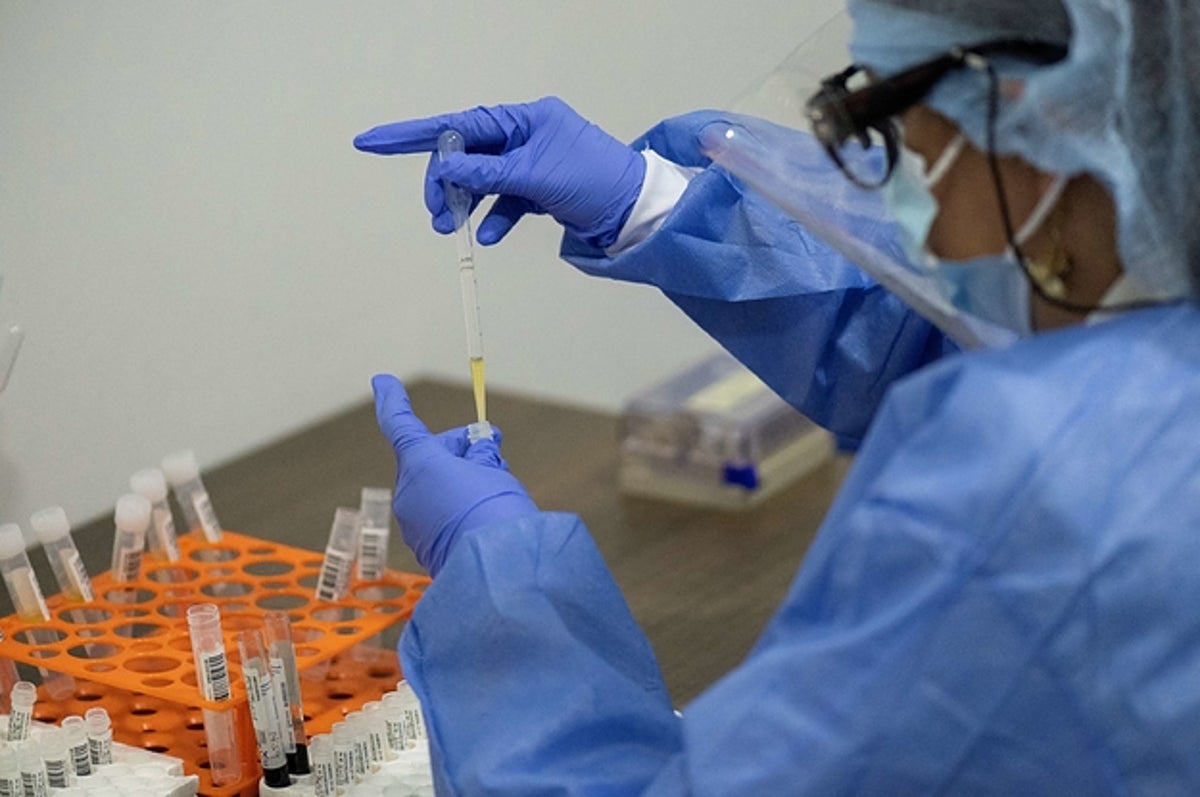
Luis Robayo / Getty Images
A health worker sorts blood samples for Johnson & Johnson’s Covid-19 vaccination study in Colombia on November 26, 2020.
In a boost for US vaccination prospects, federal health officials and the drug maker Janssen Pharmaceuticals on Thursday morning reported effective early results for a single-shot COVID-19 vaccine.
Officials from Janssen, a Johnson & Johnson company, said they planned to submit the clinical trial results — a 66% overall reduction in COVID-19 cases after vaccination — to the FDA for possible authorization as a third US coronavirus vaccine in February. While still effective, the reductions are less than the results for the US’s already-authorized COVID-19 vaccines, about a 95% for Pfizer-BioNTech’s and around 94% for Moderna’s.
The vaccine was 72% effective in its US testing, and 85% effective overall in preventing severe disease, as well as completely protective against death and hospitalization.
“We believe this vaccine is for everyone,” said Janssen’s Mathai Mammen, speaking at a briefing for reporters.
Launched in September, Janssen’s clinical trial enrolled 45,000 volunteers in a clinical trial where half were given real shots and half were given placebos.
The trial was held in the US, Brazil, and South Africa, where a now-dominant variant strain proved more resistant to the vaccine, which appears equally protective across all age groups, according to Janssen.
The Janssen vaccine relies on a harmless cold virus, called an adenovirus, that has been given the genes for the spike protein used by the coronavirus to infect people’s cells. The vaccine familiarizes the immune system against the spike protein, readying it against the actual virus when it attacks.
Already used for a successful Ebola vaccine, adenovirus vaccines require less care than the two already-authorized mRNA vaccines, easing their administration in pharmacies and smaller clinics. They can be stored in normal refrigerators for up to three months.
The Janssen vaccine results come just as the numbers of US vaccine doses administered has exceeded the reported number of US COVID-19 cases reported, both around 26 million. Only in the last week have US vaccination rates begun to exceed more than 1 million shots a day.
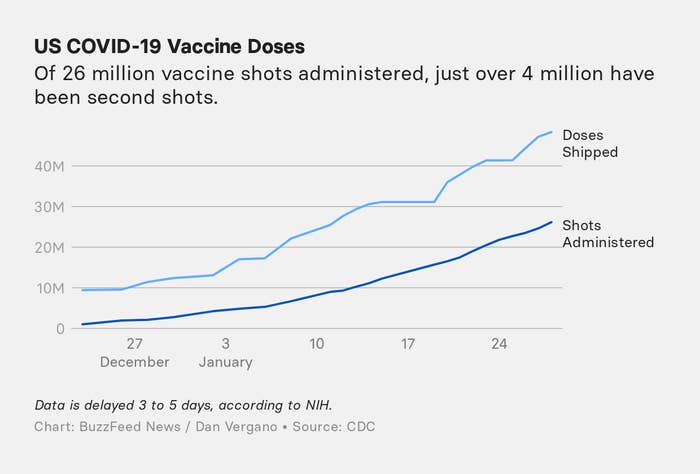
BuzzFeed News/Dan Vergano
About 10 million doses of the currently-authorized two-shot vaccines are now being shipped nationwide every week. It was originally hoped that Janssen would be able to add another 12 million shots to US distribution in February, if authorized by the FDA, but earlier this month, Operation Warp Speed’s Moncef Slaoui, said the company would deliver only a few million due to manufacturing delays, reportedly 3 to 4 million shots. Janssen is under contract to deliver 100 million shots by the end of June, and under terms of an agreement that brought the company $1 billion in federal manufacturing assistance, another 300 million shots can be contracted for by the US government.
On Wednesday, the Novavax vaccine candidate also supported by Operation Warp Speed, the $18 billion vaccine and drug-development private-public partnership, reported early results from clinical trials in the United Kingdom, where it reported an 80% drop in COVID-19 cases among its vaccinated participants, and a 60% reduction among HIV-negative participants in its trial in South Africa (it produced a 49% reduction in the smaller numbers of HIV-positive participants there, still better than many flu vaccines against the flu), where a mutated, more contagious strain of the coronavirus now predominates.
Janssen Pharmaceuticals Companies / Via jnj.com
Janssen’s investigational COVID-19 vaccine
Notably, those results suggest that current vaccines designed with the spike protein from the original strain of the coronavirus will become less effective over time against newer strains such as the B.1.351 one now dominant in South Africa, which was reported on Thursday in the first US cases, in South Carolina. The threat of new, more transmissible strains of the coronavirus may force vaccine makes to manufacture booster shots for vaccines aimed against these new threats. A US clinical trial of the Novavax vaccine is expected to finish enrolling volunteers next week, with early results expected in February.
The pandemic has caused more than 54 million COVID-19 cases and killed more than 1.3 million people worldwide, with more than 246,000 of the deaths in the US. Cases are still surging across the US, killing more than 1,400 people a day. More than 1 million new cases of COVID-19 were reported in the US last week.
This is a breaking news article. Please come back for updates and follow BuzzFeed News on Twitter.
[ad_2]
Source link